Industry Sector
iCare HOME2 Self-Tonometer clears FDA
iCare HOME2 Self-Tonometer clears FDA
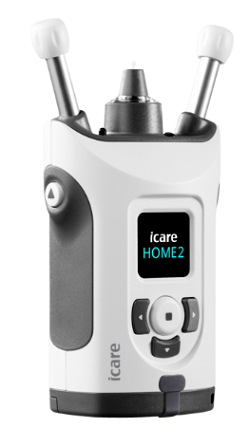
Icare USA announced 510(k) clearance from the FDA for their next generation self-tonometer, the iCare HOME2.
The iCare HOME2 tonometer provides ease of use in measuring patients’ real-world IOP outside normal clinic hours to help support glaucoma management.
“Our R&D department has made significant advancements to the iCare HOME2 tonometer,” said John Floyd, President/CEO of iCare USA. “Patients can collect real-world IOP data at any time, and with ease.”
Most patients can utilize the iCare HOME2 on their own by following the smart light guide and the interactive display screen. iCare HOME2’s new design also allows IOP measurements to be taken while the patient is supine, reclined, and sitting. It is easier than ever for doctors to customize glaucoma management based on comprehensive, real-world IOP information.
iCare CLINIC is a cloud-based software that stores IOP data measured by the iCare HOME2 tonometer. The iCare CLINIC reports provide health care professionals an in-depth overview of changes in their patients’ IOP status.
Patients can actively participate in their eye care management using the iCare HOME2 and PATIENT2 mobile app. The novel PATIENT2 mobile app allows patients to share IOP measurements more efficiently via Bluetooth using their smartphone for health care professional review. Patient motivation for medication compliance can improve, and knowledge of their real-time IOP levels provides daily reassurance.